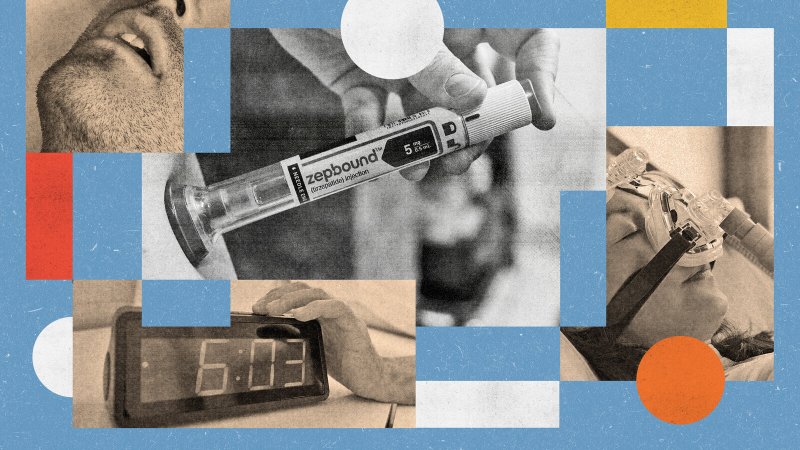
Insurance Zepbound Sleep Apnea – Eli Lilly’s Weight Loss Drug Zepbound can help treat sleep apnea – when federal supervisors expand the approved use of Zepbound with sleep apnea, releases medication compensation methods, drugs can be combined
Eli Lily announced today that the drugs behind Zepbound have been found to help relieve sleep apnea in obese patients in clinical research. This drug, Tirzepatide, is sold by a US company in the case of diabetes, as Mounjaro and for weight loss at Zepbound.
Insurance Zepbound Sleep Apnea

Pharmaceutical Giant said Wednesday it plans to request approval from the US Food Control Agency (FDA) to expand drug use in treating sleep apnea. It may also have open access to the medication. If regulators expand the approved use of Zepbound, the drug could join Novonordisk Wegovy in the fact that it is Medicare covered.
Weight-loss Drug Linked To Sleep Apnea Reduction: Research
The medication was tested in 469 participants with obesity and severe obstructive sleep apnea (OSA). This is a respiratory disorder in which the upper part of a person is completely or partially disintegrated during sleep.
As known as the CPAP machine, in the group that did not have positive respiratory pressure (PAP), the average number of participants’ breathing showed limited or full blocks of airflow after 52 weeks at 27.4 events per hour (55%).
Another group that lasted 52 weeks on PAP therapy reduced the average number of limited or blocked respiratory events by an average of 30.4 events (62.8%) per hour. Furthermore, the two groups lost average baseline weights of 18% and 20%, respectively.
“OSA has impacted 80 million adults in the US, with over 20 million living in moderate to severe OSA,” Jeff Emick, Senior Vice President of Product Development at Elilily, said in a press release. “However, 85% of OSA cases have not been diagnosed and are not treated.”
Eli Lilly’s Obesity Drug Zepbound Approved To Treat Sleep Apnea In U.s.
He added that tilzepatide may be the first drug to treat OSA, rather than treating excessive sleepiness associated with the disease.
Promising results could be a path for Zepbound to be covered by US federal health insurance. In March, the Centers for Medicare and Medicaid Services (CMS) in the United States issued new guidelines that state that “anti-obesity drugs that obtain FDA approval for extra medically accepted indications can be considered part-D medicine for their specific use.”
The guidelines were issued about a week later with American supervisors expanding the approved use of Wegovy to reduce the heart risk in obese or overweight adults. Novo Nordisk announced the results of a clinical test that reduced the risk of serious cardiac events by 20% approximately eight months ago.

DRUS’ sykrockering demand with weight loss has transformed Elirily and Novonordisk into the most valuable pharmaceutical companies in the world. Zepbound manufacturer Eli Lilly is the world’s largest company through market capitalization, with a total of $720 billion. And the sale of Wegovy and Ozempic of Novo Nordisk helped boost Denmark’s GDP in 2023.
Can Weight Loss Medications Like Zepbound Help Treat Sleep Apnea?
However, the high demand for these drugs has made it difficult for some patients to meet their recipes. The US Food and Drug Administration said in April that it was limited, along with two Zepbound and several Mounjaro diabetes medications. The FDA said there was a shortage of drug supply at least at the end of April due to increased demand.
Since last year, Novo Nordisk has restricted the starter of the Wegovy starter to ensure that patients already taking the medication have sufficient stock. But the problem is the configuration. Novo Nordisk CEO Lars Fruergaard Jørgensen said in March that the gap between supply and demand for weight loss is so important that it will take years to close. Obstructive sleep apnea (OSA) has long been treated with machinery (such as CPAP) and lifestyle changes, but now there is a new option in the mix – Zepbound for sleep apnea.
This message explores what Zepbound is, how it works for sleep apnea, the most important research behind approval and the important questions about Medicare and insurance coverage.
Who has been on the market for years. However, it is sold specifically for weight management and currently for sleep apnea.
Zepbound And Mounjaro Might Help Reduce Obstructive Sleep Apnea Symptoms
As a sleeping indented medicine clinic, everything we do, from testing to diagnosis to recipes, is online with the personal touch of having your own special sleep document.
Zepbound was originally a prescription drug developed for weight loss, and was the first drug approved by the FDA to treat moderate to severe sleep apnea in obese adults at the end of 2024 (Malhotra, 2024). This is big news for patients with OSA. Because Zepbound addresses the cause of the condition (excess weight) by helping people lose weight, which can improve their breathing at night.
It is given as a weekly injection designed to help people lose weight. It functions by activating specific intestinal hormone receptors (GLP-1 and GIP). This slows down each other’s digestion, suppressing appetite, and rather hunger (Hudgel, 2018). By reducing and reducing diet, patients grasp one of the most important factors that exacerbate sleep apnea. Successful weight loss is actually key to management
The OSA case is the kind of doctor I prescribe Zepbound as part of my sleep apnea treatment plan (US FDA, 2024). Essentially, Zepbound helps people throw pounds, and losing excess weight often leads to reduced apnea events and better sleep (Greer, 2020).
Does Medicare Cover Zepbound For Sleep Apnea?
It is noteworthy that tilzepatide, the active ingredient in Zepbound, was first used in the diabetes drug Murujaro. Both Zepbound and Mounjaro contain tilzepatides (Greer, 2013), but they are sold for a variety of purposes. Mounjaro has type 2 diabetes, and Zepbound covers obesity-related disorders such as chronic weight management and sleep apnea. By helping patients reach substantial weight loss, zepbound may indirectly improve indirectly or resolve obesity-related symptoms.
In December 2024, Zepbound was specifically FDA approved for the treatment of obstructive sleep apnea in obese adults (Malhotra, 2024). This became the first drug ever approved to treat OSA, as previously treated and surgically replaced OSA devices (such as CPAP). Approval was supported by mandatory clinical studies. especially,
Covers Zepbound or similar weight loss medications if obesity is approved (AARP, 2024). However, things changed as soon as Zepbound received approval for sleep apnea. Medicare can cover the medication if a medical condition is approved that simply exceeds weight loss. In early 2025, the Centers for Medicare & Medicaid Services (CMS) confirmed that Medicare could cover the Part D plan for the treatment of obstructive sleep apnea (AARP, 2024).
What about other insurances for Medicare? In the world of private insurance, coverage is a bit of a patchwork. Many private insurers are hesitant to cover expensive weight loss drugs such as Zepbound (Hudgel, 2018). With FDA approval for sleep apnea (which can become a serious and life-threatening condition), we start watching the shift. Insurance companies often reconsider coverage when healthcare is indicated for medical conditions as well as cosmetics and lifestyles.
The Fda Just Approved Eli Lilly’s Zepbound For Sleep Apnea — And Medicare Coverage Could Be Next
That said, coverage varies greatly depending on your insurance company and plan. There are no universal rules based on private insurance – all companies decide whether Zepbound in their state or not
(US FDA, 2024). If you’re wondering if Zepbound Insurance covers it, the best approach is to check the drug formulation in your plan or call your insurance provider directly. And depending on the case, at least a good doctor (we would like to think)
Sleep apnea Zepbound is a promising new road for the treatment of OSA and by tackling it with excess weight, which is its source. It is an FDA approved drug, showing impressive results by reducing sleep apnea and simultaneously supporting waste (Malhotra, 2024). Thanks to historical surface testing, there is solid evidence that weight loss via zepbound can often improve or resolve obstructive sleep apnea (Greer, 2020).

Previous Answer: What are the symptoms that cause sleep apnea? Second, weight loss and sleep apnea treatment (complex) The popular Elilily (LLY) weight loss drug Zepbound can be covered by a Medicare plan to treat sleep apnea after being approved by the Food and Drug Administration (FDA) last month.
Sleep Apnea Devices Market Revenue To Attain Usd 12.39 Bn By 2033
On Thursday, Zepbound, “Current Medicare Part D and Medicaid Coverage Rules apply,” will be added to the Part D plan and will be eligible for Medicare. An estimated millions of Americans suffer from obstructive sleep apnea, a sleep disorder.
Eli Lilly and Ozempic and Wegovy Maker Novo Nordisk (NVO) test the skills of weight loss pills to treat disorders that normally accompany obesity, such as diabetes and sleep apnea. The change was first reported on Wednesday
“This is good news for Medicare beneficiaries living with moderate to severe obstructive sleep apnea (OSA) and obesity, especially due to the lack of other drug treatments for underlying conditions.”
“I’m happy to see the center